n2o lewis structure
Mark lone pairs Step 3. For N2O resonance structures there is one major structure but one other resonance structure.
 |
| Resonance Theory Ochempal |
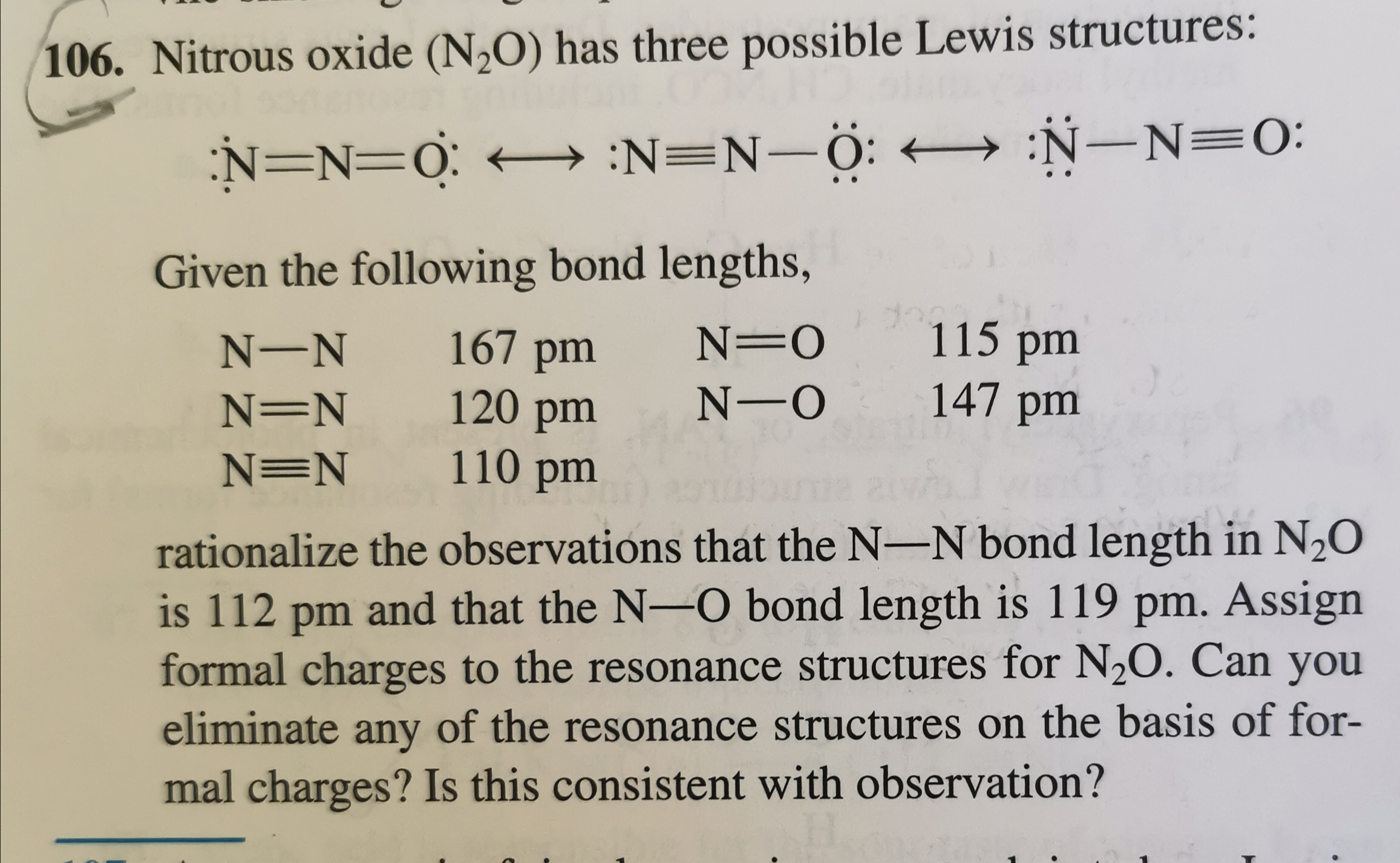
N2O lewis structure has 1 triple bond between the two Nitrogen atoms N and 1 single bond between the Nitrogen atom N and Oxygen atom O.
. N2O is also called Dinitrogen monoxide or Nitrous Oxide. So first find the element with lowest. A step-by-step explanation of how to draw the N2O5 Lewis Dot Structure Dinitrogen pentoxideFor the N2O5 structure use the periodic table to find the total. Nitrogen belongs to group 15.
Heres how you can draw the N 2 O lewis structure step by step. In the Lewis structure of N2O structure there are a total of 24 valence electrons. Mark charges Step 4. One of the 2 nitrogen atoms is present at the center of the molecule while the.
Children Book Review. There are 3 different ways to draw the Lewis structure for N2O. If a mixture of nitrogen oxide and a little oxygen is inhaled for a sufficiently long time it produces hysterical laughter. N with 3 lone pr 1 bonding pr to N with 3 bonding pr to O with 1 lone pr.
The location of the double bond changes over time meaning. In this structure more electronegative O atom bears negative charge and less electronegative N atom bears a. Nitrogen oxide is also known as laughing gas. Nv Nlp.
Draw 50 Buildings and Other Structures. Now lets find out the lewis structure of N2O The valence electrons of the atoms are- Nitrogen 5 2 Nitrogen 52 10 Oxygen 6 Total valence electrons 16 Next we need. Lewis Structure of NO2. The Step-by-Step Way to Draw Castles and Cathedrals Skyscrapers and Bridges and So Much More.
The Lewis structure of nitrous oxide N2O is made up of 2 nitrogen N atoms and one atom of oxygen O. The most stable Lewis structure of N 2O is represented by option D. We start with a valid Lewis structure and then follow these general rules. A molecule of nitrogen dioxide consists of one nitrogen atom and two atoms of oxygen.
Let us look at the periodic table. Nitrogen dioxide does not have a single Lewis structure on account of its relatively strange electron configuration. Draw sketch Step 2. Count the valence electrons of atoms.
For the Lewis structure for N2O you. The following are the steps to construct the Lewis Structure. Minimize charges Step 5. There are 3 lone pairs on both the.
The most stable of these structures has Nitrogen in the middle double bonded on one side to another ni. Thats why nitrous oxide is. N2O lewis structure formal charge The formal charge is defined as the charge over a particular molecule assuming that all the atoms have the same electronegativity. For the N2O Lewis structure we need to figure out the number of valence electronsin.
著者 ビスワラップチャンドラデイ in 化学. Nitrous oxide N2O has three valid Lewis structures.
 |
| N2o Lewis Structure Dinitrogen Oxide Ap Chemistry Nitrous Oxide Molecules |
 |
| Nitrous Oxide N2o Formal Charge |
 |
| Lewis Dot Symbols For The Representative Elements Ppt Download |
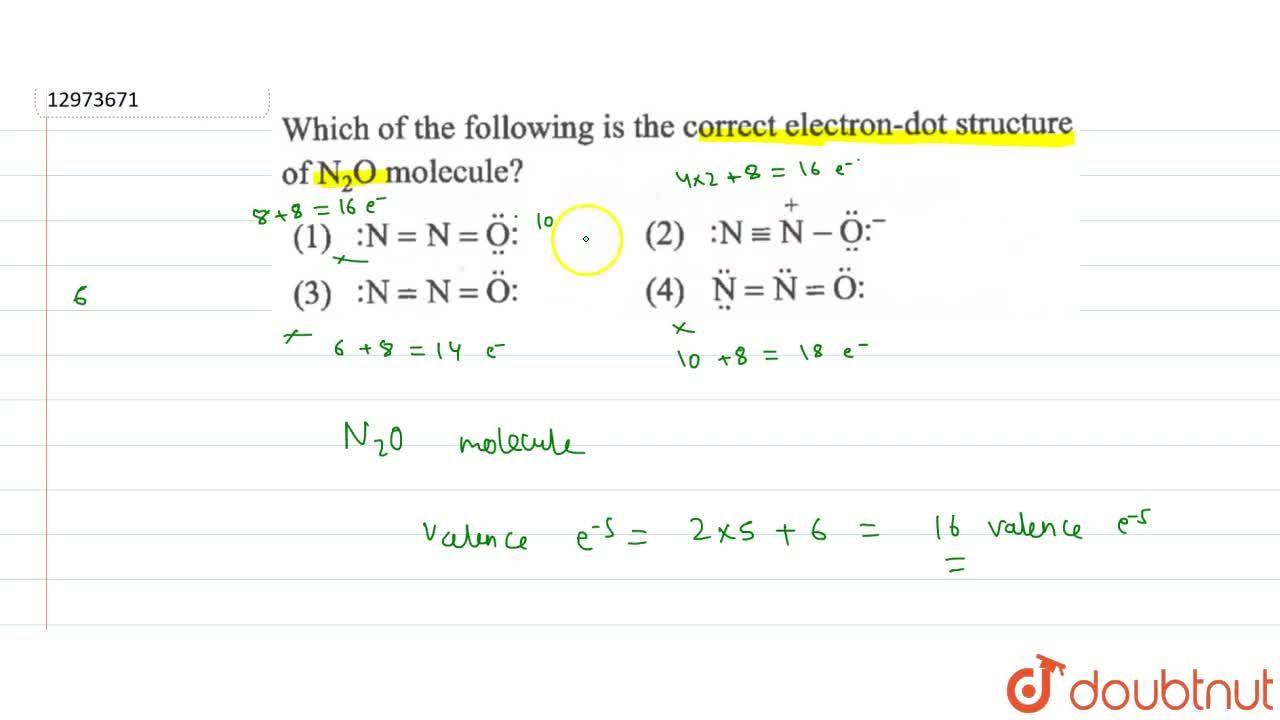 |
| Which Of The Following Is The Correct Electron Dot Structure Of N2o Molecule |
 |
| What Are The Correct Resonance Structures Of Nitrous Oxide Chemistry Stack Exchange |
Posting Komentar untuk "n2o lewis structure"